Summary of the project
Context
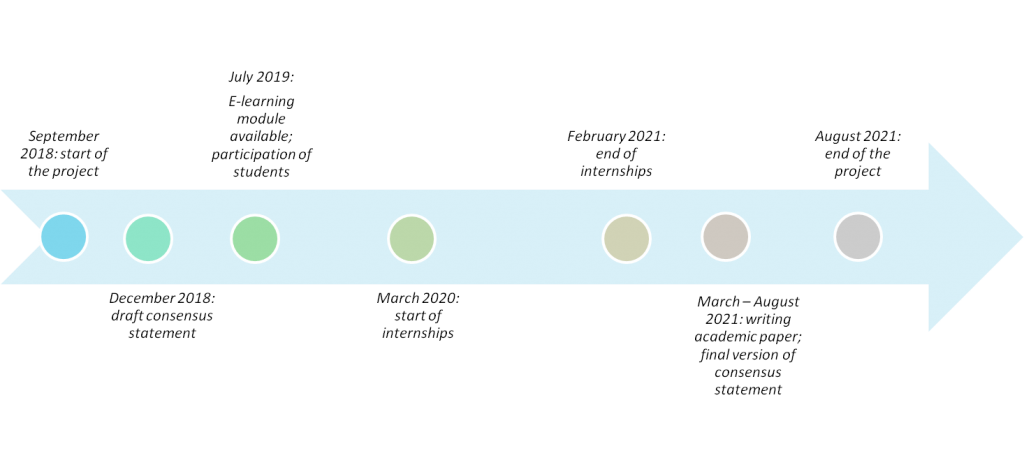
Healthcare technologies including complex technologies such as cell/gene/regenerative therapies, prenatal tests and E-health technologyies are being developed at an unprecedented rate. For a rational use of these technologies, their potential value and impact needs to be explored. Healthcare Technology Assessment (HTA) provides a systematic evaluation of the properties and (in)direct effects of healthcare technologies, aimed at informing decision making (e.g. reimbursement). Currently, HTA involves the assessment of safety, clinical efficacy and cost-effectiveness, and sometimes ethical, legal, organisational and social issues associated with the use of healthcare technologies. These issues are addressed separately from each other as distinct and potentially relevant aspects that need to be taken into account in decision making. A drawback of this approach is that it does not account for the fact that the selection of aspects that are being addressed in the HTA process crucially depends on the value framework from which the assessment is conducted.
The various stakeholders (e.g. patients, healthcare providers, payers, policy makers) often reason and act from different value frameworks in ways that affect the content and outcome of HTA. For example, the introduction of extracorporeal membrane oxygenation (ECMO), a technique to provide prolonged life support to patients whose heart and lungs are failing, has raised serious ethical dilemmas, in particular for its use in newborns. The use of ECMO is invasive and can lead to serious complications, such as paralysis, stroke, brain disorders or even death. For parents of newborns, other values and views might be important for determining whether ECMO is the right treatment for their child than for e.g. doctors or policy makers. As such, HTA should start by involving stakeholders and support them in collaboratively exploring which issues need to be addressed, how, and why. Rather than separately assessing ethical, legal and social issues as one of the aspects of a healthcare technology, we need to acknowledge that such issues provide the framework that is necessary to determine what empirical evidence is needed in order to assess a healthcare technology’s value.
Need
There is a need to steer assessment processes of healthcare technology in a more inclusive fashion. This need is driven by the fact that current HTA is insufficiently equipped to assess complex technologies, not sufficiently taking into account the value choices that are made when defining the nature of a health problem, the sort of measures that seem appropriate in resolving it and criteria that may be used to express their relative merit. Taking into account the diversity in stakeholder characteristics and preferences, context, their informational needs and ethical considerations, sets quite different demands on HTA professionals. On top of their basic knowledge and skills of traditional areas (biomedical sciences, research methodology, biostatistics, health economic modelling), HTA experts need to develop additional knowledge and skills (competences) to fulfill this role.
Objectives
The goal of the VALIDATE project is to train and introduce the next generation of HTA experts to a novel, integrative approach to HTA, wherein the study of safety, clinical and cost-effectiveness of new healthcare technologies and their wider ethical, legal and social implications are closely integrated and stakeholders are involved in a more productive way throughout the process of HTA.
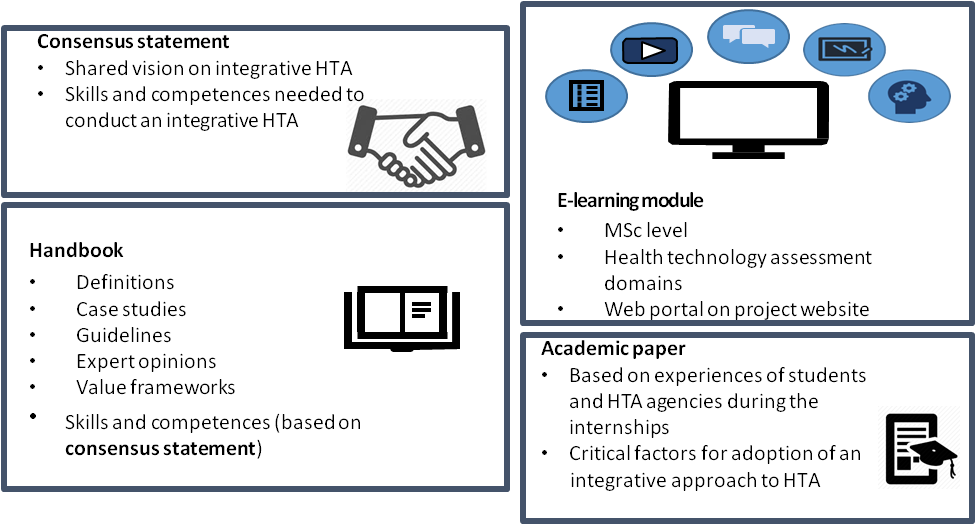
We will first develop a consensus statement on what knowledge and skills are required to conduct this novel type of integrative HTA. Based on this, an E-learning module and handbook for HTA students will be developed that help students in acquiring knowledge and skills that are needed in conducting an integrative HTA. During the VALIDATE project, students will be offered the opportunity to take this E-learning module and this will be followed, after a successful completion of the module, by an internship at an HTA agency or hospital based HTA unit. Based on the experiences of students during the participation in the E-learning module and internships, an academic paper will be written that describes the factors that are critical for the implementation of an integrative HTA process into their daily routine.
Target group
VALIDATE will train students at MSc level, enrolled in curricula on HTA, healthcare policy and management, health sciences and biomedical sciences in EU member states to become the next generation of HTA experts.
Output
The VALIDATE project aims to produce four outputs:
- Consensus statement
- E-learning module
- Handbook for integrative HTA
- Academic paper
Timeline